EFFICACY STUDIED IN A PHASE III CLINICAL TRIAL
CAMCEVI was evaluated in an open-label, single arm, multinational study involving 137 patients with advanced prostate cancer with a baseline morning serum testosterone level of >150 ng/dL and ECOG performance status of ≤2.1
CAMCEVI was initially dosed subcutaneously, 42 mg on Day 0 and again at Week 24.1
≤50 ng/dL
Serum testosterone by Week 4 through Week 48 of treatment after the initial injection.1
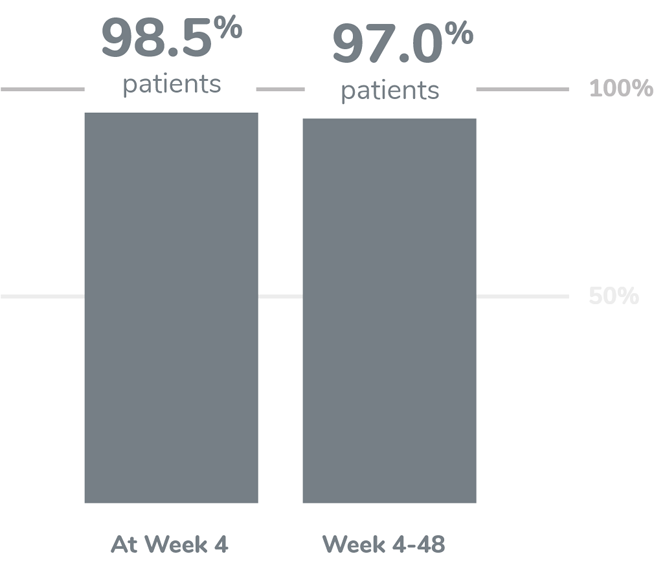
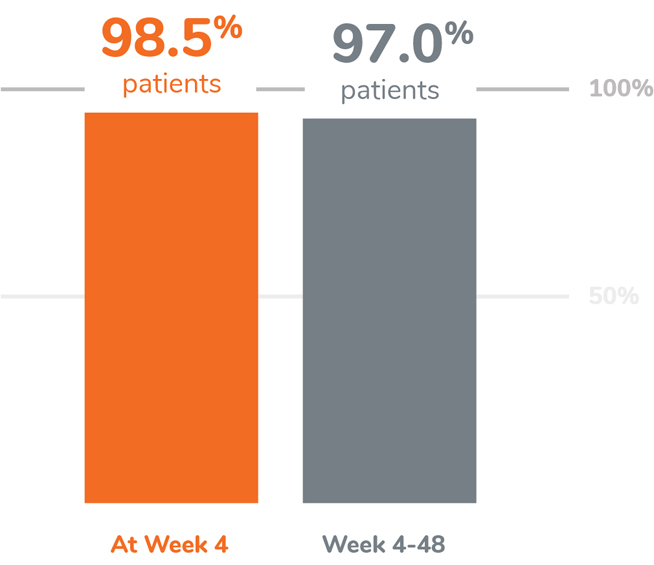
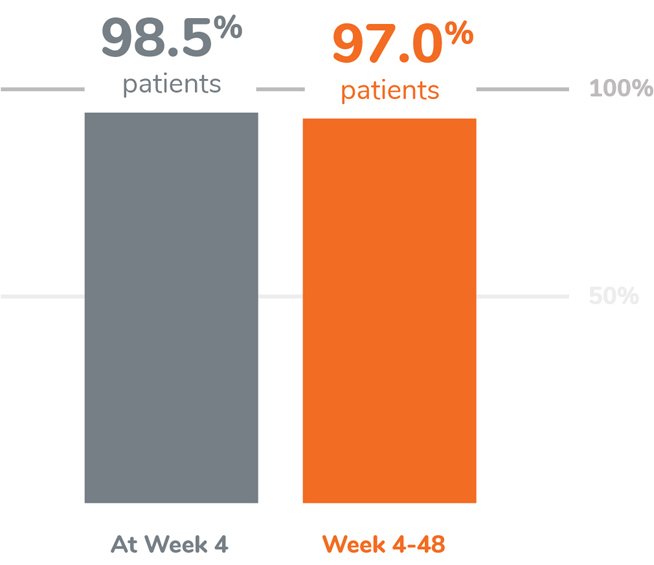
KEY FINDING: EFFECTIVE SUPPRESSION OF TESTOSTERONE TO THE CASTRATION LEVEL
KEY FINDING: EFFECTIVE SUPPRESSION OF TESTOSTERONE TO THE CASTRATION LEVEL
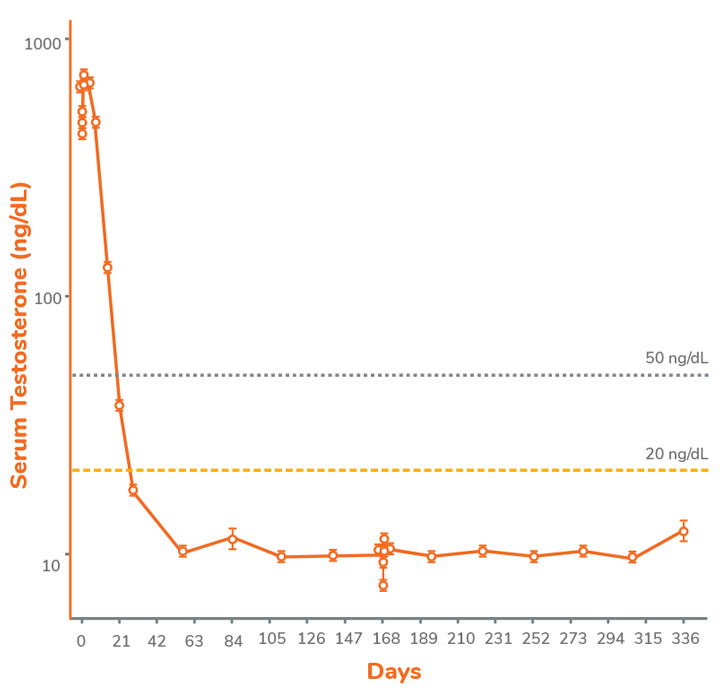
Following the first injection of CAMCEVI, serum testosterone levels were suppressed to ≤50 ng/dL by Week 4 (+/-7 days) in 98.5% of the patients.1
From Week 4 through Week 48, serum testosterone levels were suppressed to ≤50 ng/dL in 97.0% of patients (95% CI: 92.2-98.9) estimated using the Kaplan-Meier method.1
CONSISTENT SUPPRESSION OF TESTOSTERONE
TO CASTRATE LEVELS, AFTER INITIAL DOSE, FROM WEEK 4 TO WEEK 481
Mean Serum Testosterone in the Intention-to-Treat Population2
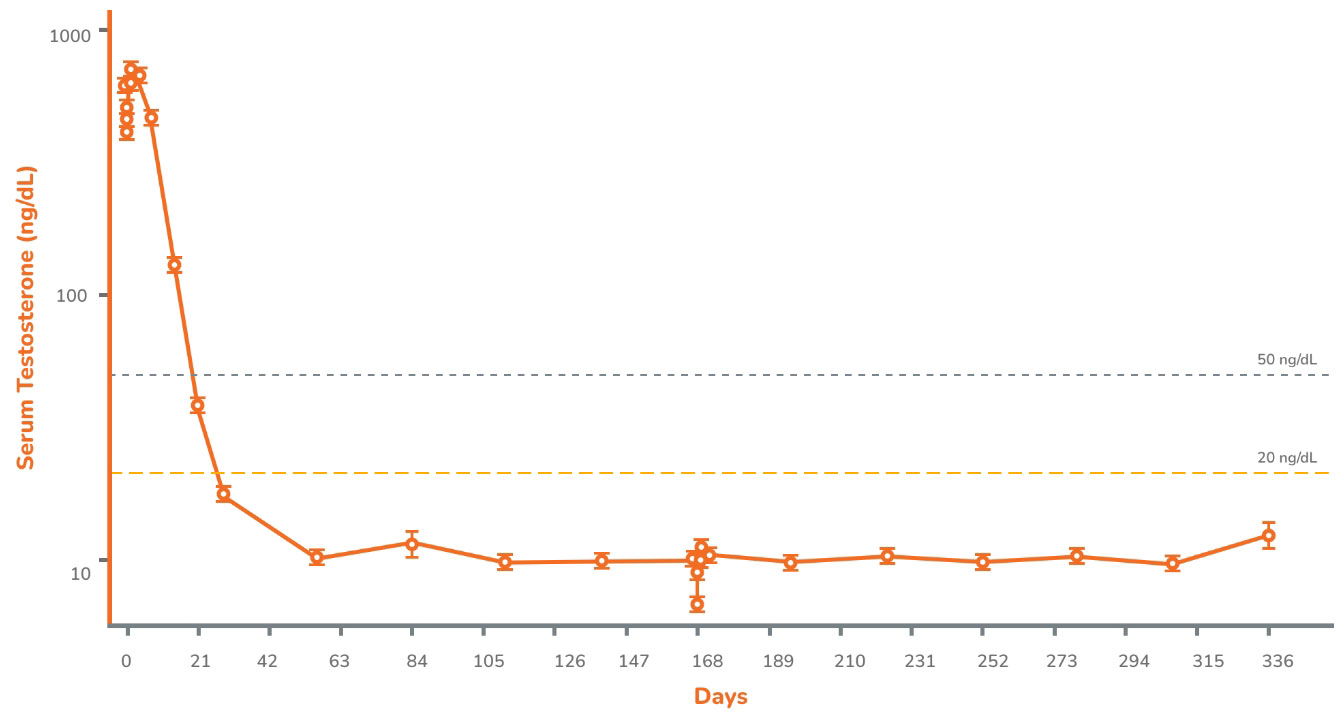
ACHIEVING PROFOUND CASTRATION1
69.3%
of patients with testosterone suppression to ≤20 ng/dL on Day 28.1
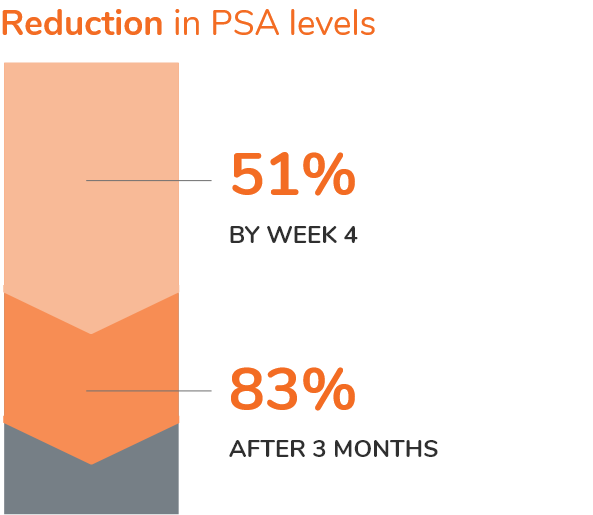
SUPPRESSION OF PSA
OVER WEEKS 4-481
PSA levels were monitored and were lowered on average by 51% after 4 weeks after administration of CAMCEVI, 83% after 3 months, and remained suppressed throughout the 48 weeks of treatment.1
These PSA results should be interpreted with caution because of the heterogeneity of the patient population studied. No evidence has shown that the rapidity of PSA decline correlates with clinical benefit.
PHARMACOKINETIC CHARACTERISTICS1
Leuprolide concentration is variable, exhibiting an initial rapid increase followed by a rapid decline over the first 3 days before reaching steady concentrations for the duration of the dosing interval.1
Following the first and second doses
Mean serum leuprolide Cmax was 94.5 and 99.0 ng/mL, respectively1
Mean serum concentration was maintained at 0.497-2.57 and 0.507-2.39 ng/mL after Day 31
Mean AUC0-6 mon was
224 and 268 day ng/mL1
SUB-Q ADMINISTRATION WITH ZERO GRADE 3-4 INJECTION-SITE REACTIONS1
CAMCEVI® is delivered subcutaneously and had an injection site reaction rate of just 11%* with no grade 3-4 injection site reactions reported in the efficacy study—allowing patients to focus more on their lives than on their advanced prostate cancer treatment.1
*Includes injection site pain, injection site erythema, injection site hemorrhage, injection site nodule, injection site paraesthesia, injection site pruritus, and injection site warmth.1




DON’T SWEAT SYRINGE PREP
CAMCEVI comes prefilled with no mixing required—simplifying syringe prep for you.1
Don’t sweat
the coverage2,3
*As of 02/01/24.
Coverage is not indicative of
utilization management criteria.
96%
patients covered by
Medicare*
91%
patients covered by
Commercial*
*As of 02/01/24.
Coverage is not indicative of
utilization management criteria.